
High-Throughput Oligonucleotide Synthesizer

The most versatile high-throughput
oligonucleotide synthesizer

Multiple Drain System
Our patented design allows the user to drain selective rows or columns at a time, with no vacuum system needed to drain the plates or columns, providing for a faster, more cost effective, consistent and stable environment throughout the run.
This selective draining feature allows the user to run 16 individual protocols in parallel, massively increasing efficiencies. Optimization of synthesis protocols can be performed in a single run, testing up to 16 different conditions without having to worry about run-to-run variability.
The use of positive pressure to purge isolated pathways means it is no longer necessary to dispense ACN to each unused well, there is no longer a need to stop to allow the entire plate to drain, and the operator can select as few as 6 columns to drain at a time in the 96 well configuration, or a single row in the 192 configuration, for greater efficiencies, more consistent and faster run times, and higher yields.
Multiple Protocol System
Multiple Protocol System
With access to 16 dedicated drain pathways, the user can now engage 16 protocols simultaneously within one run. This means 6 columns for each of the 16 conditions in the 96 well configuration, or one row for each condition in 192 configuration. All amidite and reagent handling can be made specific to each protocol. This includes injection order, injection volume, reaction time, and Pre/Post-synthesis handling procedures.
Multiple scales can be run simultaneously on the same plate. Any user-defined range is allowable. The instrument can accommodate any quantity of support that will fit within a standard high-throughput synthesis column.
Safety and Reliability
The Shasta has been engineered with an understanding of the highly reactive chemistry necessary to synthesize oligos. Every piece with direct exposure to fluids or vapors is made from and/or reinforced with PTFE, Kalrez®, PEEK, or Stainless Steel. All fluidic and electronic pathways are shielded from the user. Parts subject to replacement are made easily accessible
to minimize down-time.

Built to UL’s Standards, the Shasta has passed all testing to achieve compliance with UL’s current laboratory safety regulations, and it is the first oligo synthesizer in over 20 years to do so. All Shasta instrumentation is regularly inspected and approved by UL’s regulatory commissioners.
As with all Sierra BioSystems products, the Shasta comes with customer-site installation, a software and synthesis training package, and a one-year warranty, which includes parts and labor. Full Factory Service and Preventative Maintenance contracts are available.
Features and Consumables
Features

Standard offerings on Shasta
instruments include:
- 24 Amidite Positions configured for Septum Vials or Boston Rounds per customer specification
- 8 Reagent Positions configured to accept 28/400, GL38, or GL40 threads
- End-to-end Kalrez® and PTFE seals on every delivery line
- 2 – 40nmol synthesis in typical 384 well configuration
- 2 – 1000nmol synthesis in typical 96 column configuration
- Amidite-Specific handling
- Multi-Protocol Map™ Synthesis
- Sulfurizer integration
- Positive pressure reaction chamber ensures anhydrous environment
- Full software – no license fees
- One year warranty and spare parts package
Consumables

The Shasta is designed to work with
readily available consumables:
- ABI and Biosearch style columns
- Loose CPG and Fritted columns
- Polystyrene loaded columns
- Seahorse 96 well plates
- Biocomma 96 well plates
Performance
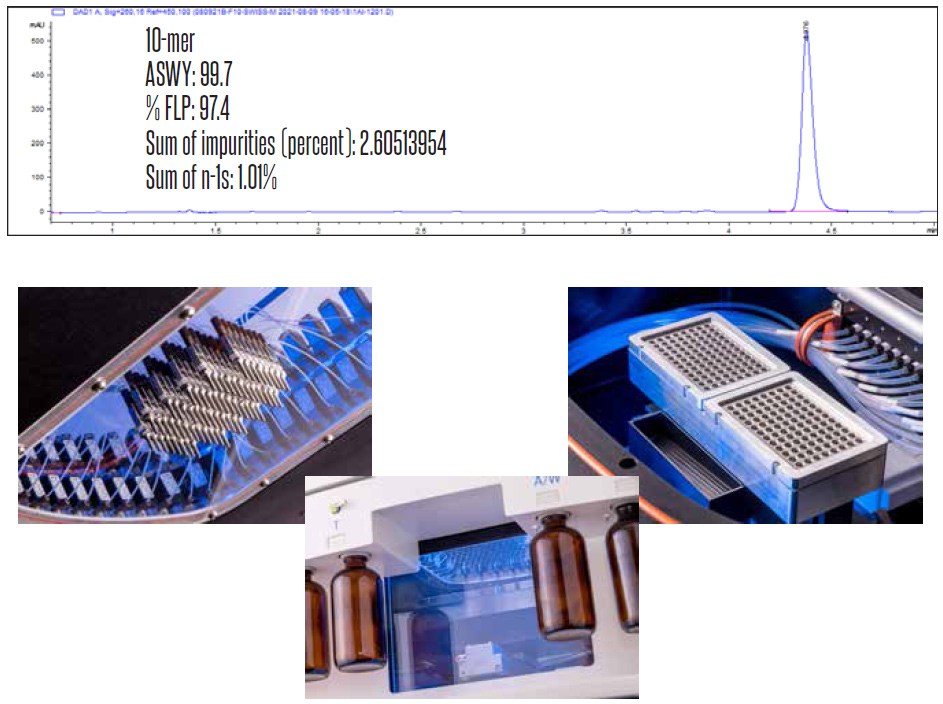
ASWY and Purity
- 99.0-99.7%+ ASWY is regularly achievable with recommended protocols and reagents, with Coefficient of Variance under 0.2%.
- Expected purity for the Shasta synthesizer is typically 90% for a 20 mer oligo.
Reagent Consumption
- Consumption per base for 0.2 μmol scale synthesis as of 9/12/2021*:
- Deblock: 300μL
- Activator: 120μL
- Amidite (50mM): 75μL
- Cap A: 50μL
- Cap B: 50μL
- Oxidizer: 100μL
- ACN: 500μL
* We continually optimize protocols to reduce consumption. Please inquire for a current usage report.
Software
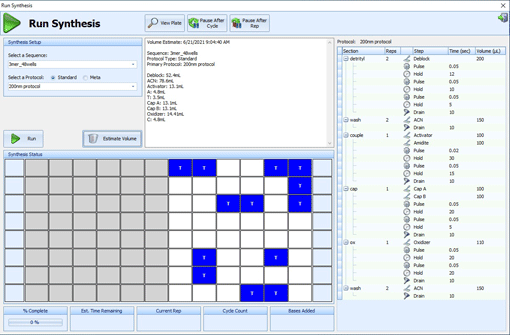
The Shasta Software is designed to offer the user an intuitive way to generate and monitor complex synthesis procedures:
- Vary protocols throughout a run with Meta™ Protocols
- Assign protocol actions beyond standard coupling procedures to each phosphoramidite
- Run multiple protocols simultaneously
- Dispense and pulse small volumes through wells at specified intervals
- Offer control over every valve and injection type
- Assign any liquid to any valve and optimize movement patterns accordingly
- Real-time synthesis monitoring for all operations
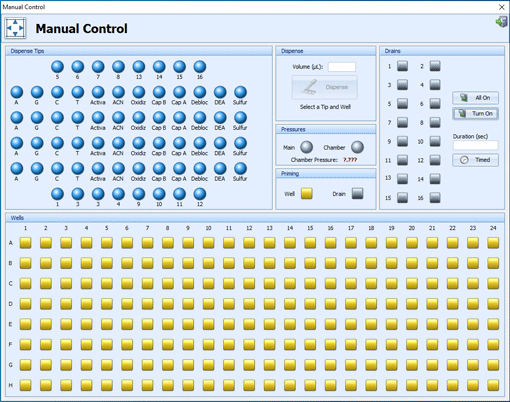
At each stage, the Shasta allows maximum control over all synthesis functions while safeguarding against potential operator error:
- Stop individual syntheses without interrupting the overall run
- Unattended operation and step-by-step plate monitoring available throughout the run
- Automatic sorting of sequences from Microsoft® Excel reduces process error
- Protocol writer proofreads protocols for errors before each synthesis
- Log files to easily source input errors
- Remote software support available
